
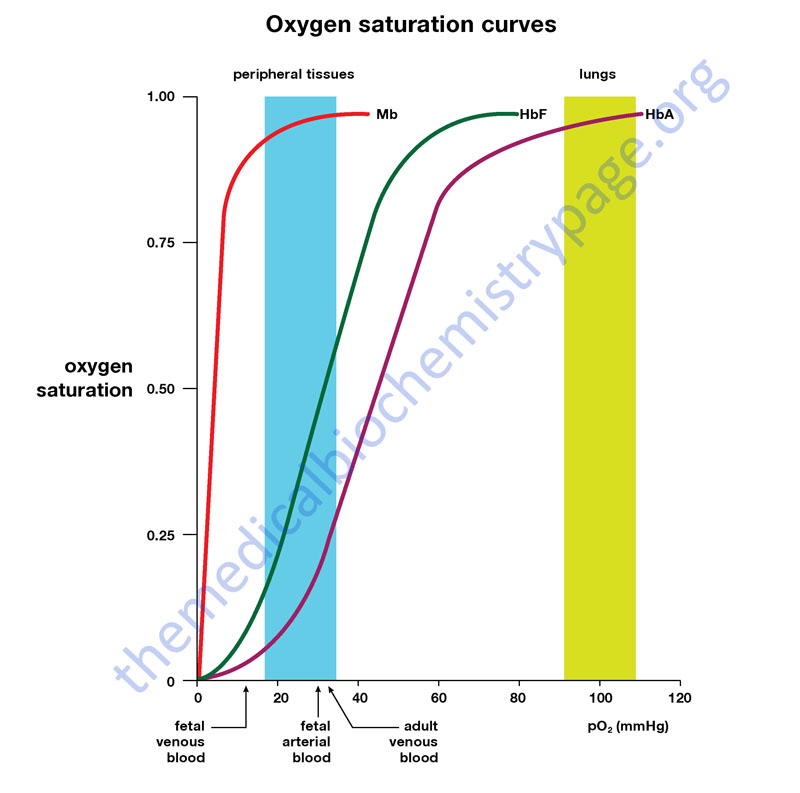
It prevents the reactive oxygen species from escaping by modifying the intrinsic reactivity of the heme group. Once the oxygen has been successfully bound, the structure of myoglobin comes into play. First, it has a proximal histidine group that helps it bind oxygen. Myoglobin owes its high affinity for oxygen to several factors. And unlike hemoglobin which is found in the red blood cells, myoglobin is found in muscle tissues. Myoglobin's affinity for oxygen is higher than hemoglobin. This allows the oxygen that is binded to have a negative charge, which stabilizes it. However, when oxygen binds to the iron, it gets oxidized to an oxidation state of 3+. Normally, the iron group in myoglobin has an oxidation state of 2+. When myoglobin is able to bind to oxygen, it serves as the primary oxygen-carrying molecule in muscle tissue. Whether myoglobin binds to oxygen depends on the presence of the prosthetic group, heme. Myoglobin exists either in an oxygen free-form called deoxymyoglobin or in a oxygen bound form called oxymyoglobin. The globular structure of myoglobin consists mainly of alpha helices linked together by various turns. Myoglobin is a single-chain globular protein that consists of 153 amino acids and a heme group (an iron-containing porphyrin). Its functions primarily in storing oxygen and facilitating oxygen diffusion in muscle tissue. Myoglobin is a small oxygen-binding protein found in muscle cells. This nonpolypeptide unit is noncovalently bound to myoglobin and is essential for the biological activity of the protein. Within a hydrophobic cervice formed by the folding of the polypeptide chain is the heme prosthetic group. In fact there are eight alpha-helical secondary structure in myoglobin.

X-ray crystallography revealed that the single polypeptide chain of myoglobin consist entirely of alpha-helical secondary structure. Myoglobin is a typical globular protein in that it is a highly folded compact structure with most of the hydrophobic amino acid residues buried in the interior and many of the polar residues on the surface. It was the first protein to have its three-dimensional structure determined by x-ray crystallography by John Kendrew in 1957. Myoglobin is a relatively small protein of mass 17.8kDa made up of 153 amino acids in a single polypeptide chain. 2 Real world examples: How is Myoglobin used?Ĭharacteristics of myoglobin.


 0 kommentar(er)
0 kommentar(er)
